Biosimilars Market Size, Share, Price, Trends, Growth, Analysis, Report, forecast 2023-2031
- 1 Overview of Biosimilars Market
- 2 Components of the Biosimilars Market
- 2.1 Impact of COVID-19 on the Biosimilars Landscape
- 2.2 Segmenting the Biosimilars Market
- 2.3 Biosimilars: Benefits and Real-world Applications
- 2.4 Market Propellants
- 2.5 Challenges and Barriers
- 2.6 Opportunities and Market Projections
- 2.7 Trending in the Biosimilars Market
- 2.8 Biosimilars Market Analysis
- 2.9 Key Market Movers
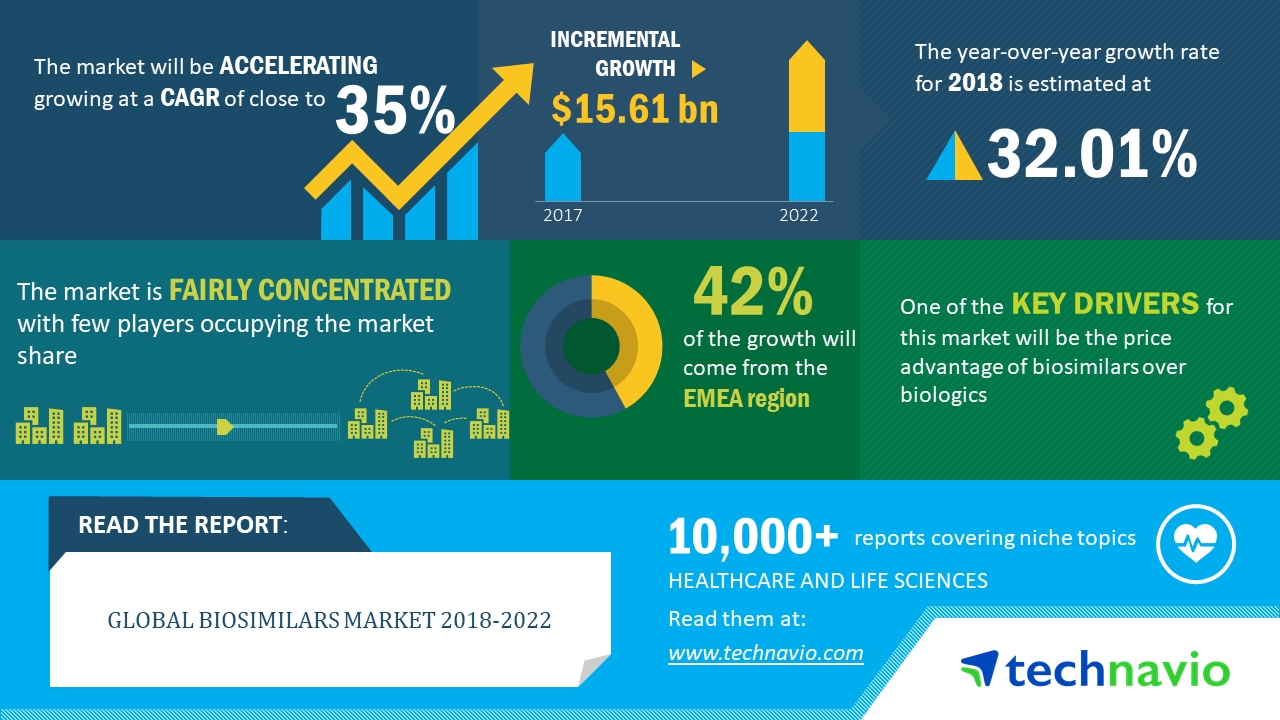
The pharmaceutical world experienced a notable shift in 2022 when the biosimilars market size reached an astounding value of USD 24.5 billion. As we progress into the coming decade, this market exhibits immense potential, set to grow at an impressive CAGR of 17.60% from 2023 to 2031. By the end of this period, it’s anticipated to burgeon to a whopping USD 105.1 billion. This meteoric rise highlights the pivotal role biosimilars play in modern healthcare and underscores their significance in the future of medicine.
Overview of Biosimilars Market
The biosimilar sector, a burgeoning segment of the pharmaceutical industry, holds the promise of revolutionizing treatments for countless patients worldwide. A biosimilar, essentially an affordable counterpart to its biological drug equivalent, promises efficiency without burning a hole in the pocket. With the escalating prevalence of chronic conditions, particularly rheumatoid arthritis among women above the age of 40, the demand for this cost-effective alternative is witnessing an unprecedented surge. Couple this with the growing global elderly demographic, and the appeal of biosimilars becomes abundantly clear.
Components of the Biosimilars Market
To truly appreciate the nuances of biosimilars, one must delve into its core components. While they aren’t direct replicas, biosimilars are engineered to emulate the parent biologic drug as closely as possible. Their design ensures they maintain similar bio-active characteristics as their licensed counterparts. The slightly modified ingredient list in biosimilars does not compromise their efficacy, safety, or efficiency, ensuring that patients receive quality care at a fraction of the cost.
Impact of COVID-19 on the Biosimilars Landscape
The relentless spread of COVID-19 has left no industry untouched, and the biosimilar market has had its share of challenges. The pandemic’s chokehold on global supply chains had a ripple effect, disrupting the seamless supply of integral components, especially Active Pharmaceutical Ingredients (APIs). Given that China, a significant supplier of APIs and the pandemic’s epicenter, faced severe setbacks, the world, especially pharmaceutical powerhouses like India, grappled with shortages. This disruption is further amplified when considering that India, which leans heavily on China for 70% of its pharmaceutical production, is a primary supplier to the U.S., catering to about 18% of its API needs.
Segmenting the Biosimilars Market
To navigate the vast expanse of the biosimilar market, understanding its segmentation is pivotal. The market’s mosaic is a rich tapestry of molecules, manufacturing methodologies, indications, and geographical expanses:
- Molecules: This includes a variety of molecules such as Infliximab, Insulin Glargine, Epoetin Alfa, and several others.
- Manufacturing: This is segmented into In-house, where biosimilars are produced within a company’s facilities, and Contract Manufacturing, where production is outsourced.
- Indications: Here, biosimilars find applications across Auto-Immune Diseases, Blood Disorders, Oncology, and other health conditions.
- Regions: The market’s geographical expanse encompasses North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
Biosimilars: Benefits and Real-world Applications
Biosimilars are more than just cost-effective pharmaceutical alternatives. Their applications span an array of conditions, including but not limited to, multiple sclerosis, inflammatory bowel disease, diabetes mellitus, and psoriasis. Their near-parity with original drugs in terms of potency and purity positions them as reliable treatment options.
Market Propellants
Several dynamics power the forward momentum of the biosimilar market. Foremost among these is the impending patent expiration of many biologics, which creates a vacuum that high-quality, approved biosimilars are primed to fill. Add to this the burgeoning healthcare expenditure in emerging economies, heightened health awareness, and lifestyle-induced diseases like diabetes, and the biosimilars market’s growth trajectory becomes clear.
Challenges and Barriers
Every industry faces its set of challenges, and the biosimilar market is no exception. Navigating the strict regulatory landscape poses a considerable hurdle. Each biosimilar must undergo rigorous testing to ascertain its efficacy, safety, and interchangeability, often leading to significant time and financial investments.
Another barrier is the initial reluctance among healthcare professionals. Their concerns revolve around the safety and interchangeability of biosimilars with their biological counterparts. Convincing these frontline health workers of the biosimilar’s merits is crucial, as their endorsement plays a pivotal role in patient adoption.
Moreover, market competition is fierce. With multiple players vying for a slice of the biosimilars pie, establishing a foothold becomes an uphill battle. Ensuring consistent quality, maintaining affordability, and staying abreast of evolving regulations become day-to-day challenges for biosimilar producers.
Opportunities and Market Projections
The silver lining amid these challenges is the vast potential the biosimilar market holds. With increased R&D investments, the coming decade promises a slew of advanced biosimilars that could redefine treatment methodologies. As global awareness and acceptance rise, biosimilars stand on the cusp of becoming the preferred treatment choice for various ailments.
Trending in the Biosimilars Market
Current market trends underscore the relentless R&D by pharmaceutical behemoths, all vying to introduce enhanced efficacy biosimilars at competitive prices. With an uptick in FDA approvals, the market’s expansion seems unstoppable. Europe, with its avant-garde biosimilar production facilities, emerges as a significant market influencer.
Biosimilars Market Analysis
Analyzing the biosimilars market using tools like SWOT and Porter’s Five Forces Models reveals a market teeming with opportunities and challenges. Strengths include its cost-effectiveness, rising global demand, and increasing FDA approvals. Weaknesses encompass the rigorous approval processes and the reluctance among some healthcare professionals. Opportunities lie in patent expirations and increased R&D. Threats include stringent regulations and intense competition. Navigating these dynamics requires strategic planning and foresight.
Key Market Movers
Dominating the biosimilars market are heavyweights like Pfizer Inc., Celltrion Inc., and Novartis AG. Their strategic moves, encompassing expansions, mergers, and R&D investments, provide intriguing insights and shape the future of biosimilars.